The systemic exposure of methotrexate from Otrexup PFS at doses of 10 15 20 and 25 mg was higher than that of oral methotrexate by 17 13 31 and 36 respectively. It is also indicated for severe recalcitrant disabling psoriasis.
Antares Atrs Buy 4 00 Why I Think That The Otrexup Launch Will Be A Success Paid Subscribers Only Expert Financial Analysis And Reporting Smith On Stocks
You may need frequent blood tests for monitoring when taking Otrexup methotrexate.

Otrexup vs methotrexate. Methotrexate is available in a number of different dosage forms including subcutaneous injection auto injector. While on Otrexup methotrexate you should avoid alcohol and staying out in the sun for too long. For patients switching from oral methotrexate to Otrexup consider any differences in bioavailability between oral and subcutaneously administered methotrexate see Clinical Pharmacology 123.
Otrexup methotrexate drug interactions. 30 mgweek should not ordinarily be exceeded. Methotrexate is used to.
Neoral cyclosporine is good at preventing rejection of a transplanted organ and is also used for treating rheumatoid arthritis and psoriasis. Methotrexate is a prescription medicine used to treat certain types of cancer severe psoriasis and severe rheumatoid arthritis. Because methotrexate has been reported to cause fetal death andor congenital anomalies Otrexup Rasuvo and RediTrex are contraindicated in pregnant women.
There are 589 drug interactions with Otrexup methotrexate Otrexup methotrexate disease interactions. Dosages may be adjusted gradually to achieve an optimal response. The first FDA-approved auto-injectable Otrexup is marketed by.
OTREXUP methotrexate injection was recently approved by the US. Methotrexate systemic absorption from Otrexup was similar when administered into the abdomen or thigh. Prescribed As Part Of A Treatment For Psoriasis And Rheumatoid Arthritis.
Methotrexate systemic absorption from Otrexup PFS was similar when administered into the abdomen or thigh. Treat certain adults with severe active rheumatoid arthritis and children with active polyarticular juvenile idiopathic arthritis pJIA after treatment with other medicines including non-steroidal anti-inflammatory drugs NSAIDS have been used and did not work well. While on Otrexup methotrexate you should avoid alcohol and staying out in the sun for too long.
The systemic exposure of methotrexate from Otrexup at doses of 10 15 20 and 25 mg was higher than that of oral methotrexate by 17 13 31 and 36 respectively. Benefits of these new products include convenience and ease but the drawback is a much higher cost. MTX Amethopterin and Methotrexate Sodium are other names for Methotrexate.
Rasuvo and Otrexup are prefilled auto-injection devices that allow patients to give themselves subcutaneous injections of methotrexate without having to use a separate syringe and vial. For patients switching from oral methotrexate to Otrexup consider any differences in bioavailability between oral and subcutaneously administered methotrexate see Clinical Pharmacology 123. Reduces inflammation and cell replication.
Otrexup methotrexate can cause serious birth defects so women should avoid getting pregnant for at least 6 month and men 3 months after taking it. Use in women of child bearing age without a reliable form of birth control is not recommended 1-3. It is indicated for adults with severe active rheumatoid arthritis or children with active polyarticular juvenile idiopathic arthritis who have failed first line therapies.
Otrexup methotrexate is the first-choice treatment for many types of cancer and arthritis but it has many side effects. Food and Drug Administration. Otrexup methotrexate can cause serious birth defects so women should avoid getting pregnant for at least 6 month and men 3 months after taking it.
OTREXUP is a single-dose auto-injector containing a prescription medicine methotrexate. The different brands of methotrexate are Otrexup Rasuvo RediTrex Trexall and Xatmep. There are 5 disease interactions with Otrexup methotrexate which include.
Another auto-injectable form of methotrexate waits in the wings. Otrexup is an injectable prescription drug that is used to treat adults with severe active rheumatoid arthritis and children with active polyarticular juvenile idiopathic arthritis pJIA. In some cases health care professionals may use the trade names Otrexup Rasuvo Rheumatrex and Trexall or other names MTX Amethopterin and Methotrexate Sodium when referring to the generic drug name Methotrexate.
Last month the Food and Drug Administration FDA approved Medacs formulation called Rasuvo the first to be available in so many different dose options 10 of them ranging from 75 mg to 30 mg in 25 mg increments. Dosage may be gradually adjusted to achieve optimal clinical response. Prolonged use of methotrexate can cause hepatic fibrosis and cirrhosis.
 Otrexup Injection Launched For Ra Psoriasis Mpr
Otrexup Injection Launched For Ra Psoriasis Mpr
 Otrexup Methotrexate Injection Uses Dosage Side Effects Interactions Warning
Otrexup Methotrexate Injection Uses Dosage Side Effects Interactions Warning
 Otrexup Dosage Rx Info Uses Side Effects
Otrexup Dosage Rx Info Uses Side Effects
 Auto Injectable Methotrexate New Treatment Option For Ra The Rheumatologist
Auto Injectable Methotrexate New Treatment Option For Ra The Rheumatologist
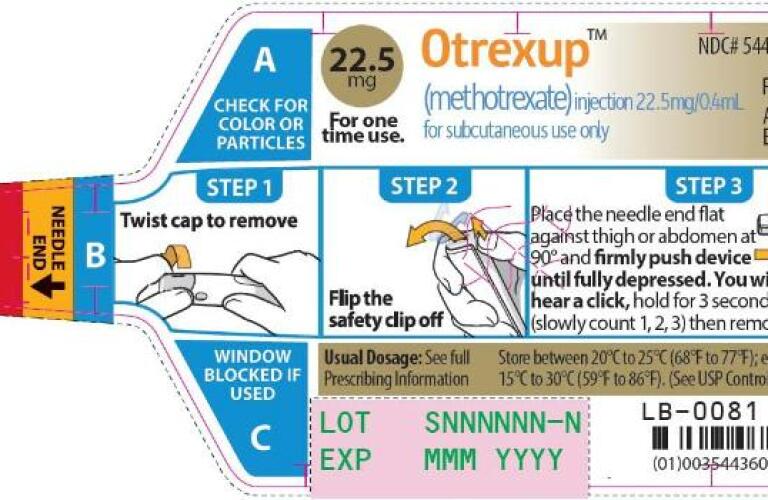
 These Highlights Do Not Include All The Information Needed To Use Otrexup Safely And Effectively See Full Prescribing Information For Otrexup Otrexup Methotrexate Injection For Subcutaneous Useinitial U S Approval 1953
These Highlights Do Not Include All The Information Needed To Use Otrexup Safely And Effectively See Full Prescribing Information For Otrexup Otrexup Methotrexate Injection For Subcutaneous Useinitial U S Approval 1953
 Otrexup Methotrexate Injection For Subcutaneous Use
Otrexup Methotrexate Injection For Subcutaneous Use
 Otrexup Methotrexate Injection Uses Dosage Side Effects Interactions Warning
Otrexup Methotrexate Injection Uses Dosage Side Effects Interactions Warning
 About Otrexup Methotrexate Injector
About Otrexup Methotrexate Injector
 Antares Pharma Inc 54436002004 Mckesson Medical Surgical
Antares Pharma Inc 54436002004 Mckesson Medical Surgical
 Otrexup For Healthcare Professionals
Otrexup For Healthcare Professionals
 Otrexup Methotrexate Injection Approved By The Fda Johns Hopkins Arthritis Center
Otrexup Methotrexate Injection Approved By The Fda Johns Hopkins Arthritis Center



No comments:
Post a Comment
Note: Only a member of this blog may post a comment.