Generally FDA requires two successful trials for a specific peripheral neuropathic pain condition eg diabetic peripheral neuropathy For a general indication of treatment of peripheral. April 16 2021 Today the US.
 Managing Chronic Diabetic Peripheral Neuropathy In The Elderly
Managing Chronic Diabetic Peripheral Neuropathy In The Elderly
Get a COMPLETE EVALUATION and take advantage of a NEUROPATHY.

Fda approved neuropathy treatment. PAID CONTENT Vero Neuropathy offers unique treatments that can heal nerve pathways and basically restore the nerves of patients with chronic pain. Pregabalin and duloxetine are the only medications approved by the US. Eli Lillys treatment for pain also used for depression Cymbalta duloxetine Pfizers treatment for epilepsy Lyrica pregabalin and JJs chronic pain medication Nucynta ER tapentadol which was approved just this past week to treat neuropathy we will have more on this late-breaking story in the next issue.
The FDA approved an 8 capsaicin patch for the treatment of adults with neuropathic pain associated with diabetic peripheral neuropathy of the feet according to an industry press release. The recommended dose range of LYRICA for the treatment of neuropathic pain associated with spinal cord injury is 150 to 600 mgday. Food and Drug Administration has approved the first use of a medicated patch made with capsaicin the spicy substance that makes chili peppers hot as a treatment for diabetic neuropathy.
Qutenza from Averitas Pharma the US subsidiary of the German pharmaceutical company Grünenthal is a cutaneous patch that delivers prescription-strength capsaicin 8 directly to. Currently there are two primary medications available to treat the pain and numbness of diabetic neuropathy. The FDA recently approved duloxetine hydrochloride Cymbalta Eli Lilly for the treatment of the pain associated with diabetic peripheral neuropathy.
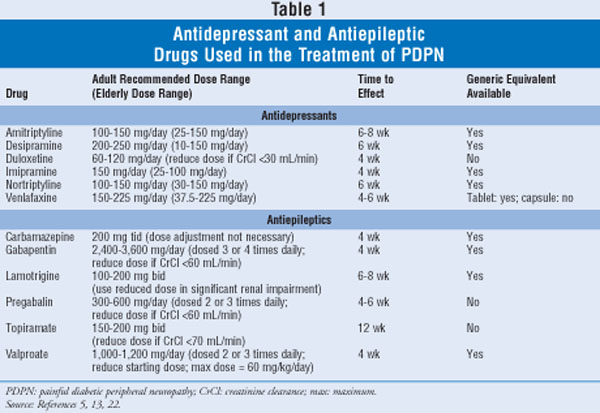
Based on current practice guidelines these medications with. 76 Zeilen The following list of medications are in some way related to or used in the treatment of this. The Qutenza skin patch is made by Grünenthal and contains 8 capsaicin which acts on pain.
PFE today announced that the US. Food and Drug Administration for treating this disorder. Food and Drug Administration FDA has approved the supplemental Biologics License Application sBLA for PANZYGA.
However many other medications are commonly used successfully to treat diabetic neuropathy. August 29 2012 The US Food and Drug Administration FDA has approved tapentadol extended-release ER Nucynta Janssen Pharmaceuticals Inc for the management of neuropathic pain associated. This is the first drug approved by the FDA.
The FDA has approved two medications for diabetic peripheral neuropathy. In early August the number of treatment. NEW YORK-- BUSINESS WIRE--Pfizer Inc.
QUTENZA capsaicin 8 patch is approved in the US for the treatment of neuropathic pain associated with postherpetic neuralgia and for the treatment of neuropathic. Food and Drug Administration FDA has approved the first generic versions of Lyrica generic name pregabalin a popular medicine used for nerve pain from neuropathy nerve damage as well as seizures fibromyalgia and other neurological conditions. The recommended starting dose is 75 mg two times a.
In what could be called a case of fighting fire with fire the US. Food and Drug Administration approved Opdivo nivolumab in combination with certain types of chemotherapy for the initial treatment. Their treatments work at the cellular level and help relieve pain and improve a patients range of motion and mobility WITHOUT drugs injections or surgery.
FDA approved a new capsaicin drug for treating diabetic peripheral neuropathy DPN of the feet in July 2020. Peripheral neuropathy could be reversed by FDA-approved class of drugs Scientists from the University of Manitoba and UCSD found that a class of already-approved drugs reversed peripheral. For Immediate Release.
Talk to you doctor to find out if these medications may be right for your nerve pain.
