However a review of outcomes of women exposed to subcutaneous interferon beta 1a during pregnancy revealed normal live births 38. Limited published literature has described the presence of interferon beta-1a products in human milk at low levels.
Https Www Accessdata Fda Gov Drugsatfda Docs Label 2002 Ifnbbio111202lb Pdf
CONTRAINDICATIONS PRECAUTIONS Subcutaneous administration.

Interferon beta 1a contraindications. Tell your doctor if you have ever had. Interferon beta 1a is contraindicated for pregnant women and should not be used unless the potential benefit justifies the potential risk to the fetus. It is not known if interferon beta-1a is excreted in breast milk.
Find out what health conditions may be a health risk when taken with interferon beta-1a intramuscular. Interferon beta-1a is an immunologic adjuvant that is FDA approved for the treatment of multiple sclerosis. As many drugs enter breast milk and can potentially cause harm to the nursing infant interferon beta-1a should be used cautiously in nursing mothers.
Angina cardiac arrhythmias cardiac disease heart failure myocardial infarction. Each 02 mL 02 cc of REBIF contains 88 mcg of interferon beta-1a. AVONEX interferon beta-1a is indicated for the treatment of relapsing forms of multiple sclerosis MS to include clinically isolated syndrome relapsing-remitting disease and active secondary progressive disease in adults.
Adult Indications and Dosage FDA-Labeled Indications and Dosage Adult. Confusion and paresis and impaired renal function. Subcutaneous administration of interferon beta-1a should not be substituted for.
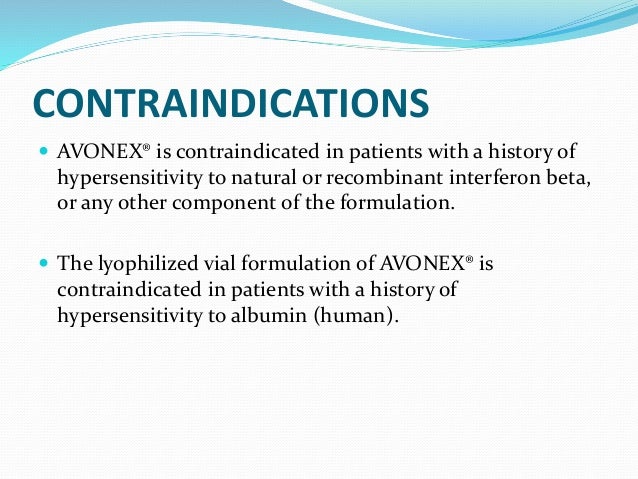
On the basis of evidence from clinical trials contraindications to the use of interferon INF are pregnancy epilepsy and depression. 1996 RECENT MAJOR CHANGES Indications and Usage 1 072019 Dosage and Administration Important Administration Instructions 22 032020 Contraindications 4 032020 INDICATIONS AND USAGE AVONEX is for the treatment of relapsing forms of multiple. Common adverse reactions include flu-like symptoms including chills fever myalgia and asthenia.
There are no data on effects of interferon beta-1a on milk production Therefore developmental and health benefits of breastfeeding should be considered along with mothers clinical need for therapy and any potential adverse effects on breastfed infant from drug or from underlying. Interferon beta-1a is classified as FDA pregnancy risk category C animal studies show an adverse effect on the fetus. Management of multiple sclerosis during pregnancy is a difficult issue because of pregnancy-related complications and fear of congenital anomalies due to exposure to disease-modifying therapy.
AVONEX 30 micrograms contains approximately. Each 05 mL 05 cc of REBIF contains either 22 mcg or 44 mcg of interferon beta-1a 2 mg or 4 mg albumin human 273 mg mannitol 04 mg sodium acetate and water for injection. Manufacturer advises patients should be monitored for clinical features of thrombotic microangiopathy TMA including thrombocytopenia new onset hypertension fever central nervous system symptoms eg.
You should not use interferon beta-1a if you are allergic to natural or recombinant interferon beta or albumin. Alcoholism hepatic disease. WebMD provides common contraindications for interferon beta-1a intramuscular.
REBIF interferon beta-1a is formulated as a sterile solution in a prefilled syringe or REBIF Rebidose autoinjector intended for subcutaneous sc injection. Bleeding problems or a blood clot. Interferon beta-1a should be used.
The rates of spontaneous abortion and major congenital anomalies in live births were in line with those observed in. Any signs of TMA should be investigated fully and if diagnosed interferon beta should be. AVONEX interferon beta-1a injection for intramuscular injection Initial US.
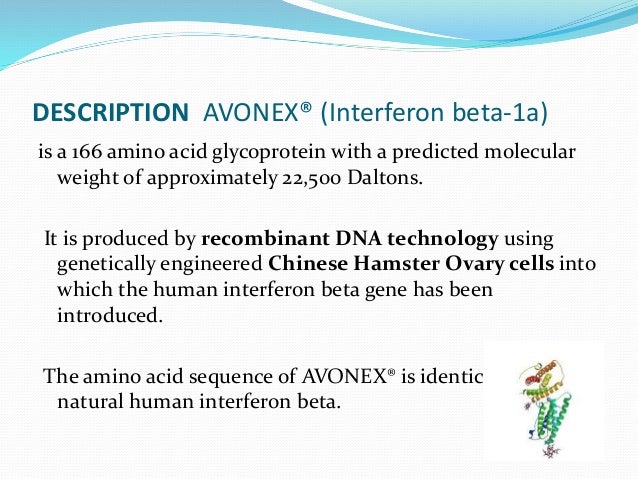
Using the World Health Organization WHO International Standard for Interferon AVONEX has a specific activity of approximately 200 million international units of antiviral activity per mg of interferon beta-1a determined specifically by an in vitro cytopathic effect bioassay using lung carcinoma cells A549 and Encephalomyocarditis virus ECM. Notably data from 2 smaller cohort studies suggest that exposure to interferon beta products during pregnancy may be associated with a decrease in mean birth weight however those findings have not been confirmed in larger observational studies.
Https Www Merckneurology Com Content Dam Web Healthcare Biopharma Neurology Merckneurology Docs Rebiff Pi Pdf
 Pdf Oral Interferon Beta 1a In Relapsing Remitting Multiple Sclerosis A Double Blind Randomized Study
Pdf Oral Interferon Beta 1a In Relapsing Remitting Multiple Sclerosis A Double Blind Randomized Study
 Congenital Anomalies Detected In Live Birth Pregnancies Exposed To Ifn Download Table
Congenital Anomalies Detected In Live Birth Pregnancies Exposed To Ifn Download Table
 Interferon Beta 1a Uses Interactions Mechanism Of Action Drugbank Online
Interferon Beta 1a Uses Interactions Mechanism Of Action Drugbank Online
 Treatment Algorithm For Non Responders To Interferon Beta Or Glatiramer Download Scientific Diagram
Treatment Algorithm For Non Responders To Interferon Beta Or Glatiramer Download Scientific Diagram
Https Tutorials Atitesting Com Assets Pdf Module2 Immunomodulators Interferonbeta 1a Pdf
 Interferon Beta 1a Side Effects
Interferon Beta 1a Side Effects
 Pdf A Prospective Study Comparing Injectable Interferon Beta 1a Rebif 22 44 Avonex 30 And 1b Betaseron 250 Injection Site Reaction In Multiple Sclerosis Patients
Pdf A Prospective Study Comparing Injectable Interferon Beta 1a Rebif 22 44 Avonex 30 And 1b Betaseron 250 Injection Site Reaction In Multiple Sclerosis Patients
 Interferon Beta 1a Serious Side Effects
Interferon Beta 1a Serious Side Effects
 Interferon Beta 1b Contraindications
Interferon Beta 1b Contraindications
 Plegridy Peginterferon Beta 1a Dosage Uses Side Effects Emedz
Plegridy Peginterferon Beta 1a Dosage Uses Side Effects Emedz


No comments:
Post a Comment
Note: Only a member of this blog may post a comment.