To purchase drugs at the 340B price covered entities must meet the following ongoing requirements. Pursuant to section 340Ba1 of the Public Health Service Act and the 340B Ceiling Price and Civil Monetary Penalty final rule 82 FR 1210 January 5 2017 the 340B ceiling price for a covered outpatient drug is equal to the Average Manufacturer Price AMP from the preceding calendar quarter for the smallest unit of measure minus the Unit Rebate Amount URA.
The 340B Drug Pricing Program For more than 25 years the 340B Drug Pricing Program has provided financial help to hospitals serving vulnerable communities to manage rising prescription drug costs.

340b drug pricing program requirements. Assessed CMPs would be in addition. Recertify eligibility every year. For more than 25 years the 340B Drug Pricing Program has provided financial help to hospitals serving vulnerable communities to manage rising prescription drug costs.
To purchase drugs at the 340B price covered entities must meet the following ongoing requirements. Here are some resources available. OPAs website provides information on all areas of the 340B Program.
Today the Health Resources and Services Administration HRSA which oversees the 340B Drug Pricing Program directed six drug manufacturers to comply with 340B pricing requirements or risk financial penalties. In a declared emergency an abbreviated health record may be adequate for purposes of the 340B Program. If the covered entity will bill Medicaid for drugs purchased under 340B then the entity must provide OPA with the Medicaid billing number.
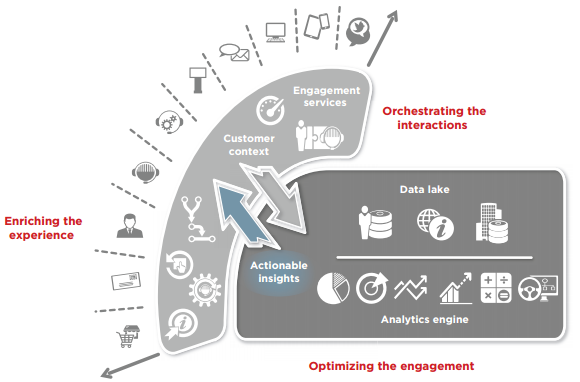
What is the 340B program. 340B Drug Pricing Program covered entities must ensure program integrity and maintain accurate records documenting compliance with all 340B Program requirements. Register new outpatient facilities and contract pharmacies as they are added.
Section 340B a 1 of the Public Health Service Act PHSA requires that the Secretary of Health and Human Services the Secretary enter into a pharmaceutical pricing agreement PPA with each manufacturer of covered outpatient drugs in which the manufacturer agrees to charge a price for covered outpatient drugs that will not exceed an amount determined under the statute 340B ceiling. Section 340B of the Public Health Service Act requires pharmaceutical manufacturers participating in. However the context of the situation may be taken into account in determining whether an individual can qualify to receive 340B drugs during an emergency and meet the patient definition as outlined by HRSA.
Resources cover registration and implementation requirements compliance program integrity and more. Register new outpatient facilities and contract pharmacies as they are added. To understand the 340B drug pricing program 340B program one must begin in 1990 when Congress created the Medicaid drug rebate program MDRP to lower the cost of pharmaceuticals reimbursed by state Medicaid agencies.
340B Drug Pricing Program covered entities must ensure program integrity and maintain accurate records documenting compliance with all 340B Program requirements. HRSA has the authority to audit covered entities for compliance with 340B Drug Pricing Program 340B Program requirements 42 USC 256b a 5 C. Under the 340B Drug Pricing Program if the State claims a Medicaid rebate for the same covered outpatient drug from the manufacturer.
For more than 25 years the 340B Drug Pricing Program has provided financial help to hospitals serving vulnerable communities to manage rising prescription drug costs. An individual receiving 340B drugs must be a patient of the covered entity as defined by HRSA PDF - 32 KB. Section 340B of the Public Health Service Act requires.
The 340B Program Ceiling Price and Civil Monetary Penalties final rule states that any manufacturer participating in the 340B Program that knowingly and intentionally charges a covered entity more than the ceiling price for a covered outpatient drug may be subject to a Civil Monetary Penalty CMP not to exceed 5000 for each instance of overcharging. Keep 340B OPAIS information accurate and up to date. The 340B program requires prescription drug manufacturers to provide discounts to specified federally-funded clinics and certain hospitals otherwise known as covered.
Under section 340B a 1 of the Public Health Service Act manufacturers of covered outpatient drugs that participate in the 340B Drug Pricing Program 340B Program must offer all covered outpatient drugs at no more than the 340B ceiling price to a covered entity listed on HRSAs public 340B database if such drug is made available to any other purchaser at any price. HRSAs Office of Pharmacy Affairs OPA is committed to educating and informing 340B Drug Pricing Program stakeholders about the program. Section 340B of the Public Health Service Act requires pharmaceutical manufacturers participating in Medicaid to sell outpatient drugs at discounted prices to health care organizations that care for many uninsured and.
Congress created the 340B program in 1992 to help certain healthcare safety net providers that serve a large number of uninsured or vulnerable patients reduce outpatient prescription drug costs. Recertify eligibility every year. Keep 340B OPAIS information accurate and up to date.
AHA along with five other provider groups in December filed a federal lawsuit against HHS alleging the department failed to enforce 340B program requirements and allowed actions from drug.